FAQ's: Glass Bottom Dishes
Dish Utilization
Mattek’s glass bottom dishes are available uncoated or coated with poly-d-lysine or collagen. All dishes are gamma irradiated to insure sterility.
A general procedure for their use follows.
- Maintain sterility: Open dishes in a sterile environment (e.g. laminar flow hood).
- Pre-equilibrate dishes: Incubate the dishes with culture medium. Pipet 2-3 ml of medium into the 35 mm dishes or 3-4 ml into the 50 mm dishes and incubate at 37° C for 15 minutes.
- Add cell suspension to microwell: Remove the culture medium by aspiration and plate cells onto the glass surface. Pipet 250 µl of the cell suspension (cells suspended in culture medium) into the 10 mm diameter microwells, 500 µl of cell suspension into the 14-mm microwells, or 1 ml of cell suspension into the 20-mm wells. Incubate the dishes for 1 hour at 37° C.
- Add additional medium: After 1 hour, gently fill the remainder of the dish with medium. Add 2-3 ml to the 35 mm dishes or 3-4 ml for the 50 mm dishes.
Note: After the initial one hour period to allow cells to attach to the glass surface, it is important to fill the dish to normal levels in order to minimize the effects of evaporation and to avoid inducing changes in osmolarity.
It is hard to predict which type of glass bottom dishes (uncoated, poly-d-lysine coated, or collagen coated) will work best with your specific cell type. Many transformed or cancerous cell lines will grow well on uncoated dishes. Poly-lysine coated dishes work well for neuronal culture and for many primary cells; other cells prefer a collagen coating. Also, many researchers purchase our uncoated dishes and apply their own specialized coating. You can also Google search for published methods utilizing glass bottom dishes and your cells (see below).
Note: Since almost all microscope objectives are optimized for use with No. 1.5 coverslips, the No. 1.5 coverslip thickness will produce the highest quality images.
We have collected and catalogued thousands of technical papers that cite the use of Mattek’s Glass Bottom Culture Dishes. You can search our database of cataloged publications on our website.
Note: If the exact glass bottom dish part number is unspecified in a literature article, it is safe to assume that uncoated dishes were used (e.g. Part #’s: P35G-1.5-14-C, P50G-1.5-30-F, etc.)
Although our poly-d-lysine or collagen coating works well for the vast majority of cells, some customers find it necessary to coat the glass bottom dishes themselves. They purchase the uncoated dishes and use the following hydrochloric acid (HCl) pretreatment along with their coating of choice.
- Under sterile conditions, pipette 250 µl of 1 N HCl onto non-coated 10-mm glass bottom dishes (e.g. Part #’s: P35G-x-10-C or P50G-x-10-F); use 500 µl or 1 ml of 1 N HCl for the 14-mm and 20-mm glass bottom culture dish, respectively (e.g. Part #’s: P35G-x-14-C or P35G-x-20-C).
- After 15 minutes, decant the HCl and rinse the dish 3x with phosphate buffered saline (PBS) and 2x with ultrapure H2O.
- Apply the coating to the dishes.
- Add a similar volume of the medium in which you will plate your cells to pre-equilibrate the glass surface. Incubate the medium in glass bottom dishes for 15 min at 37°C. Remove the medium and then plate your cells.
To try a sample of non-coated glass bottom dishes, go to our free sample page.
Yes, but for most applications, cells growing in the glass bottom dish can be viewed without removal of the coverslip using a variety of inverted and upright microscopes.
If necessary (e.g. for long term storage purposes), the coverslip can be removed using the following procedure:
- Order Part # PDCF OS 30 (Fluid for removal of coverslips from glass bottom dishes)
- Invert the cover of the dish.
- Pipette 1.0 ml of fluid into the inverted cover.
- Place the bottom of the dish onto the cover. Make sure that the liquid in the inverted cover is touching the bottom of the coverslip.
- Allow the dish to sit in the fluid for 45 minutes at room temperature.
- Dry the bottom of the coverslip with an absorbent paper towel.
- Place the dish on a clean surface. Using forceps, press down on the inner edge of the coverslip to separate the coverslip from the dish.
Note: If the above procedure is followed, the PDCF OS 30 fluid will not contact the cells and will not disrupt cells on the coverslip or the staining thereof. Coverslips can be removed without breakage.
In order to approximate physiological conditions, the temperature of the medium contained within the glass bottom dishes can be controlled by using a microscope stage heater and an appropriate stage adapter.
For use with the P35G dishes (Corning 35 mm dishes): Culture dish heaters (part#: DH-35), microscope stage adapters (part#: SA-microscope type), heater controller (part#: TC324-B), and connecter cable (part#: CC-28) are available from Warner Instrument Corporation. Information is available online at: https://www.warneronline.com/
The gridded coverslips allow one to refer to specific cells and follow them over time. For instance, individual cells can be microinjected, returned to the incubator, and observed at multiple time points since each cell can be identified with a unique alpha-numeric coordinate in the dish. Glass bottom dishes containing gridded Bellco Glass coverslips are available. Standard gridded dishes are designated as part #’s: P35G-1.5-14-CGRD and P50G-1.5-14-FGRD.
Grid size: The grid on part #’s P35G-1.5-14-CGRD and P50G-1.5-14-FGRD consists of 520 unique alphanumeric squares. Each square measures 600 μm x 600 μm. The line thickness is 20 μm.
Visualization of the grid: The grid should be readily visible using a 10X brightfield objective. After locating the cell of interest, simply switch to a higher magnification or fluorescence objective. The grid will not be visible using higher magnification or fluorescence objectives.
I can’t see the grid: A confluent monolayer of cells will typically mask the grid making it difficult or impossible to visualize. If your cells are confluent, you can utilize Part # P35G-1.5-14-CGRD-D which positions the grid on the outside of the dish where it is unaffected by the cells growing on the coverslip. To use this dish, find a cell of interest and then focus down to the bottom side of the coverslip to get its coordinates.
Gridded coverslips (18 mm x 18 mm, No. 1.5 thickness, non-sterile, part# PCS-1.5-1818-GRD) are available for purchase.
Mattek’s Glass Bottom Dishes can also be used to image tissue slices. The slices are adhered to the dishes using Corning Cell-Tak. A research paper utilizing Mattek’s glass bottom dishes to perform confocal microscopy on brain slices is available.
We do NOT recommend re-using the glass bottom dishes. The surface properties of the substrate on which cells are cultured have a profound effect on cell structure and function. Re-use of dishes will introduce uncontrolled variables into your experiments which may affect experimental results.
Mattek’s Glass Bottom Dishes are intended for single-use experiments.
Microscopy Types and Techniques
Yes, Mattek’s Glass Bottom Dishes are excellent for both confocal and fluorescent microscopy. Dishes with No. 1.5 glass thickness are preferred (e.g. P35G-1.5-14-C or P50G-1.5-14-F). Important glass properties are:
- Incident ultraviolet rays with wavelengths longer than 320 nm do not cause fluorescence.
- Mercury lines at 334 and 365 nm do not create auto-fluorescence. (Note: For mercury illumination, filter out the mercury lines with wavelengths shorter than 313 nm to obtain best possible results.)
- Refractive index (@ 20°C): nd= 1.5230 tolerance ± 0.0015
- Abbe number V = 55.
You will need to order BOTH a glass bottom dish and a glass cover. Order any P35G dish (e.g. part #’s: P35G-1.5-14-C or P35G-1.5-20-C) along with a glass cover (part #: P35GTOP-0-20-C). Both the glass bottom and glass cover are necessary so that the entire light path travels only through high optical quality glass. The glass covers can be re-used following resterilization by soaking them in 70% ethanol for 30 minutes. The covers CANNOT be autoclaved.
Yes. For high resolution techniques such as GSDIM, dSTORM, PALM, TIRF, and for any objective with a numerical aperture > 0.7, tight control of the coverslip thickness is crucial. Therefore, use of part # P35G-0.170-14-C dish which utilizes a high tolerance No. 1.5 coverslip (0.170 ± 0.005 mm) is optimal.
The 50 mm glass bottom dishes (part #’s beginning with P50G-) are useful for:
Microinjection: The larger diameter (50 mm) and the lower side wall (9 mm) allows easier access to cells in microinjection experiments.
Atmosphere maintenance: The 50 mm dish has a cover that snaps onto the dish bottom and thereby prevents loss of the 5% CO2 atmosphere while the dish is out of the incubator. This can be important for experiments in which dishes will be observed for extended periods.
Automate Scientific and Warner Instruments, Inc., make perfusion adapters which are compatible with Mattek’s 35 mm series of glass bottom dishes (Part #: P35G-xx-xx-C).
Dish Properties
Almost all microscope objectives are optimized for a No. 1.5 coverslip thickness. Use of the No. 1.5 thickness gets increasingly important for higher numerical aperture coverslips (NA > 0.7). Other coverslip thicknesses will lead to optical distortion and loss of resolution. Therefore, for the vast majority of microscopy applications, the No. 1.5 coverslip thickness is optimal (e.g. Glass bottom dish Part #s: P35G-1.5-xx-C, P50G-1.5-xx-F, P60G-1.5-xx-F, etc.).
For super-high resolution microscopy techniques, we offer glass bottom dishes with high tolerance No. 1.5 coverslips designated in part #s as -0.170 (e.g. part #: P35G-0.170-14-C).
The actual thickness of the glass coverslips depends on the Coverslip No./part #, as follows:
| Coverslip No./Part# | Thickness (mm) |
| 0 | 0.085-0.13 |
| 1.0 | 0.13-0.16 |
| 1.5 | 0.16-0.19 |
| 2.0 | 0.19-0.23 |
| 0.170* | 0.165-0.175 |
*Refers to Mattek designation in glass bottom dish Part #’s: P35G-0.170-14-C.
High tolerance, No. 1.5 thickness coverslips (170 ± 5 µm) are also available (part# PCS-170-1818).
Although the specific identity of the adhesive is proprietary, the adhesive used is a non-toxic silicone that is compatible with a broad variety of cells including primary neurons and many other difficult-to culture, fastidious cells.
Coated glass bottom dishes can be stored in the dark at room temperature for up to 1 year. Uncoated dishes can be stored for up to 3 years without any decline in cell growth properties.
The glass bottom dishes can be used over the temperature range -20°C to +45°C. The dishes will become disfigured at 55°C (131°F) and above. The glass bottom dishes CANNOT be autoclaved.
Mattek uses the highest quality, borosilicate German glass coverslips in our glass bottom dishes. The coverslip properties are as follows:
- Highest hydrolytic resistance (hydrolytic class 1).
- Excellent resistance to chemicals.
- Emission of alkali approximately 15 to 24 µg Na2O/g glass.
- Excellent properties for fluorescent microscopy.
- Incident ultraviolet rays with wavelengths longer than 320 nm do not cause fluorescence.
- Mercury lines at 334 and 365 nm do not create auto-fluorescence.(Note: For mercury illumination, filter out the mercury lines with wavelengths shorter than 313 nm
to obtain best possible results.) - Refractive index (@ 20°C): nD ** = 1.5230 tolerance ± 0.0015.
- Abbe number V = 55.
The depth of the micro-wells in the glass bottom dishes depends on the type of dish and the thickness of the dish bottom as follows:
- Corning 35 mm: 0.70-0.75 mm
- Falcon 50 mm: 1.00-1.10 mm
- Falcon 60 mm: 1.15-1.20 mm
- Falcon 100 mm: 0.90-1.00 mm*
- Falcon 6-well plate: 1.45-1.55 mm
- Falcon 12-well plate: 1.45-1.55 mm
- Falcon 24-well plate: 1.10-1.20 mm
- Falcon 96-well plate: 1.05-1.25 mm
The body of the glass bottom dishes and multi-well plates is made from polystyrene. Therefore, they have limited compatibility with organic solvents. Please see the chemical compatibility table.
| Solvent | Chemical Compatibility |
| Acetone | Poor |
| Ammonium hydroxide (1N) | Fair |
| Ammonium hydroxide (25%) | Fair |
| Aniline | Good |
| Butanol | Good |
| Chloroform | Poor |
| Dimethylformamide | Poor |
| Dimethylsulfoxide(DMSO) | Poor |
| DMSO/H2O (20/80) | Good |
| Dioxane | Poor |
| Ethanol | Good |
| Hexane | Poor |
| Hydrochloric acid (25%) | Good |
| Hydrochloric acid (concentrated) | Fair |
| Methanol | Good |
| Methyl ethyl diketone | Poor |
| Methylene chloride | Poor |
| Nitric acid (25%) | Poor |
| Nitric acid (concentrated) | Poor |
| Sodium hydroxide | Good |
| Toluene | Poor |
| Xylene | Poor |
All glass bottom dishes are gamma irradiated at an FDA approved and certified vendor. We sterilize our dishes in bulk and typically >5000 separate cases are sterilized at the same time. Since sterility is an absolute requirement for all of our customers, the gamma dose that we use is excessive in order to ensure sterility. Following sterilization, dishes are subjected to quality control analysis to verify sterility: they are incubated in antibiotic- and anti-fungal-free medium for 7 days. In addition, each box has a gamma irradiation indicator that turns red upon exposure to gamma rays.
The P35G-0.170-14-C dishes utilize high tolerance No. 1.5 thickness coverslips (coverslip thickness = 0.170 +/- 0.005 mm) versus the standard P35G-1.5-14-C dishes (coverslip thickness = 0.175 +/ 0.015 mm).
The P35G-0.170-14-C dishes will improve picture quality (see Figure below) versus the P35G-1.5-14-C dishes for any high numerical aperture objective used in confocal, fluorescence, GSDIM, dSTORM, PALM total internal reflection (TIRF), and other high numerical aperture objectives. For example, quantitative measurements using P35G-0.170-14-C dishes gave z-resolution of +/- 9.5% while z-resolution in the P35G-1.5-14-C dishes gave z-resolution of +/- 17.3% (n=5).
| A | 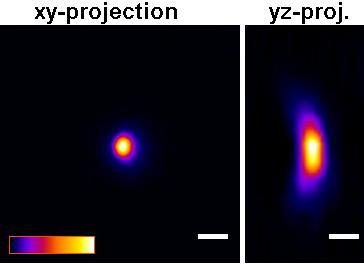 |
| B | 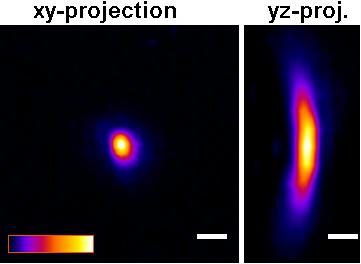 |
Figure: Improved Z-axis resolution – Effect of high tolerance glass coverslips (in P35G-0.170-14-C glass bottom dishes) on imaging of sub-resolution beads using:
A) P35G-0.170-14-C and B) P35G-1.5-14-C glass bottom dishes.
5 µl of FITC labeled 175nm PS-Speck sub-resolution beads were added to well of the glass bottom dishes and allowed to dry. After drying, 200 ul of water were added and the beads were imaged using a Zeiss LSM510 confocal microscope equipped with an Olympus UPLSAPO 60x (NA=1.2) water immersion objective.
Figures and measurements courtesy of Teemu Ihalainen, Ph. D., University of Jyvaskyla, Finland (2008).
Coatings
The poly-d-lysine used to coat the P35GC-x-xx-C and P50GC-x-xx-F glass bottom dishes is in the molecular weight range of 70,000-150,000 Daltons.
Both untreated glass and cells are negatively charged. Poly-lysine is applied to the glass surface to make it positively charged, thereby increasing electrostatic attraction between the glass surface and the cells and thus improving cell attachment. Poly-d-lysine is favored because the d-enantiomer is less prone to protease-mediated breakdown than the naturally-occurring l-enantiomer. Otherwise, Poly-d-Lysine and Poly-l-Lysine are equivalent.
The collagen used to coat the P35GCol-x-xx-C or P50GCol-x-xx-F glass bottom dishes is type 1 rat tail collagen.
The coatings are monolayer coatings which do not affect the optical properties of the glass bottom dishes.
Special Orders
Glass bottom dishes with 7-mm diameter glass surfaces are standard products (e.g. part # P35G-1.5-7-C or P35G-0-7-C). In addition, 50-mm, 60-mm- and 100-mm dishes come with a 30-mm diameter glass surface (e.g. part #P50G-1.5-30-F, P60G-1.50-30-F, P100G-1.5-30-F) and are standard products; these dishes maximize the surface area for cell growth. When very expensive reagents need to be conserved, dishes with a 5-mm diameter glass surface (e.g. part #: P35G-1.5-5-C) are also available on a special order basis.
Lead-time: Special order items typically can be produced within 2-3 weeks of receipt of a purchase order. However, bulk sterilization of the glass bottom dishes occurs only once every 6 weeks. Therefore, the lead-time for sterilized, special order products can vary between 3-8 weeks.
Note: Lead-times can be shortened if the customer will sterilize the dishes using UV and/or 70% ethanol (e.g. 40 mins under UV light in a tissue culture hood and/or immersion if 70% ethanol for 30 minutes).
Special order charges: Special order charges: For special orders of 3 cases or more, there is no special order charge; however, for special orders of less than 3 cases, a special order charge will apply.
Return policy: Special orders items cannot be returned.
Mattek Chambered Cell Culture Slides
| Product | Dimensions of wells (mm) | Medium volume/ well (mL) |
| CCS-2 | 23.0 x 22.2 | 1.2 – 2.5 |
| CCS-4 | 23.0 x 11.4 | 0.5 – 1.3 |
| CCS-8 | 11.5 x 11.4 | 0.2 – 0.6 |
Yes, cells grown on the CCS can be observed using an inverted scope. Stain your cells and remove the plastic upper chamber. Use mounting medium and coverslip the samples on the slide. Mattek coverslips, Part #: PCS-1.5-5024 (No. 1.5 thickness 50 mm x 24 mm), are specially designed for coverslipping samples cultured on the CCS.
The dimensions of microscope slide are: Length: 75.0 mm, Width: 25.0 mm, and Height: 1.0 mm.
There is a silicone gasket beneath the upper chamber and the microscope slide. The gasket typically releases from the slide when the chamber is removed. However, if the gasket remains on the microscope slide, it can easily be peeled off the microscope slide using fine point forceps. [/su_spoiler][/su_accordion]