Get the latest
Get MatTek offers and updates delivered to your inbox.
Use EpiAirway tissues to assess API permeation and flux, irritation potential and efficacy of nasal drug formulations.
METHODS
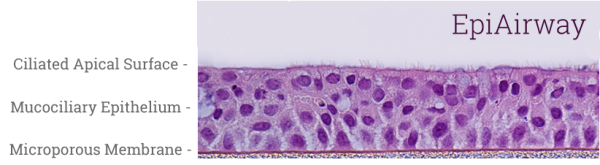
EpiAirway tissues (Figure 1) are equilibrated overnight under standard culture conditions (37°C, 5% CO2) with EpiAirway Assay Media (AIR-100-ASY). 18-24 hours later the apical surface of the tissue is washed by pipetting 300 μl DPBS up and down three times (optional). Culture supernatants are aspirated and replaced with fresh, pre-warmed media or receiver solution. EpiAirway is exposed to test formulation for up to 6 hours with permeation samples collected every 30-60 minutes. All donor and receiver samples are analyzed for API and the steady state, average flux and permeability coefficient are calculated.
As secondary endpoints, culture supernatants and EpiAirway tissues are collected for analysis of barrier integrity (Transepithelial Electrical Resistance), tissue viability (MTT Assay), cytokine/chemokine release and histological/immunohistochemical analysis.
RESULTS
Formulation optimization studies have shown that EpiAirway is highly effective for screening formulations with different permeation enhancers by evaluating barrier function, tissue viability and drug permeation. Reports demonstrate a strong correlation between EpiAirway permeation and bioavailability in various in vivo models.
CONCLUSIONS
EpiAirway’s in vivo-like barrier properties, mucociliary differentiation, drug transporters and drug metabolism enzymes make EpiAirway ideal for preclinical optimization of nasal drug formulations.
Download the Drug Delivery Protocol
For more information, view Technical References.
Learn more about EpiAirway.
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**
Get MatTek offers and updates delivered to your inbox.