Get the latest
Get MatTek offers and updates delivered to your inbox.
The in vitro EpiDerm MTT ET-50 assay is used as a screen to assign expected in vivo irritancy responses based on the time-to-toxicity (ET-50) results obtained with EpiDerm. This assay is also used to rank order test formulations according to their skin irritation potential.
During product development of new personal care or cosmetic formulations, the skin irritation potential is evaluated to identify substances which may induce adverse skin reactions. Understanding the potential of topically applied chemicals or formulations to induce skin irritation is an important consideration to avoid exposing human volunteers to potentially irritating materials during clinical testing.
MatTek offers the EpiDerm Skin Irritation Test as a non-GLP service.
Protocol
| Test Model | EpiDerm |
| Replicates | N=2 tissues per test condition |
| Exposure Time | 2, 5 and 18-hour topical exposure to 100µl or 100mg of test material per tissue |
| Assay Controls | Negative Control – diH2O Positive Control – 1% Triton |
| Endpoints | MTT Tissue Viability Assay |
| Data Delivery | ET-50 values Expected in vivo irritancy category (i.e. strong, moderate, mild, non-irritating) |
Download the EpiDerm Skin Irritation MTT ET-50 Protocol
Data
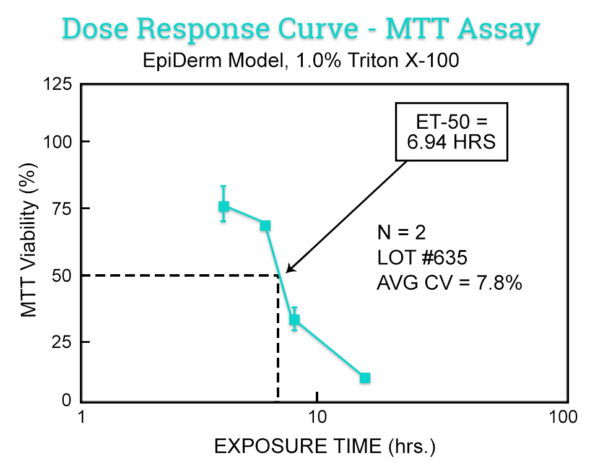
References
Reference 809 – Application of MatTek In Vitro Reconstructed Human Skin Models for Safety, Efficacy Screening, and Basic Preclinical Research
Faller, C. et al., (2002). Predictive ability of reconstructed human epidermis equivalents for the assessment of skin irritation of cosmetics. Toxicology In Vitro. 2002 October;16(5): 557-572.
Request a Quote
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**
Get MatTek offers and updates delivered to your inbox.