Get the latest
Get MatTek offers and updates delivered to your inbox.
The EpiIntestinal tissue model is useful for testing candidate drugs or biologics to treat diseases that are characterized by injuries of small intestine epithelial barrier.
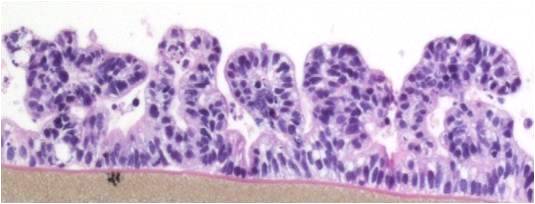
EpiIntestinal is a 3D reconstructed tissue model produced from primary, human cell-derived small intestine epithelial and endothelial cells and fibroblasts. The highly differentiated tissue model is produced at the air-liquid-interface (ALI) in easy-to-handle tissue culture inserts. Structural analysis of the tissue model demonstrates columnar shaped basal cells and Kerckring folds. Ultrastructurally, EpiIntestinal exhibits brush borders, functional tight junctions and mucous secreting granules, similar to in vivo tissue.
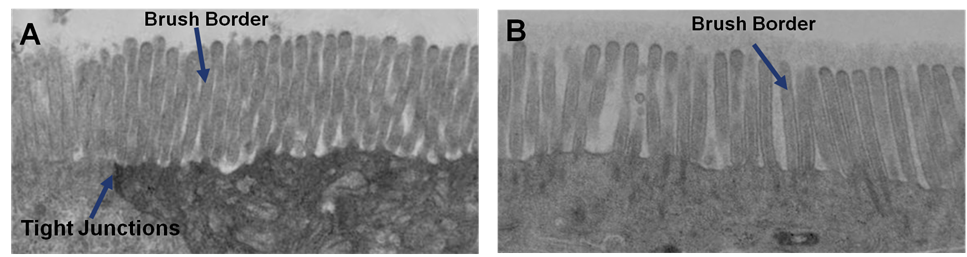
Ultrastructural Analysis: Transmission electron micrograph (TEM) of in vitro EpiIntestinal (A) and Explant tissues (B) showing Brush borders (situated at the luminal pole of the enterocyte) and tight junctions. Brush border – provides digestive and absorption surface; site for enzymes & transporters.
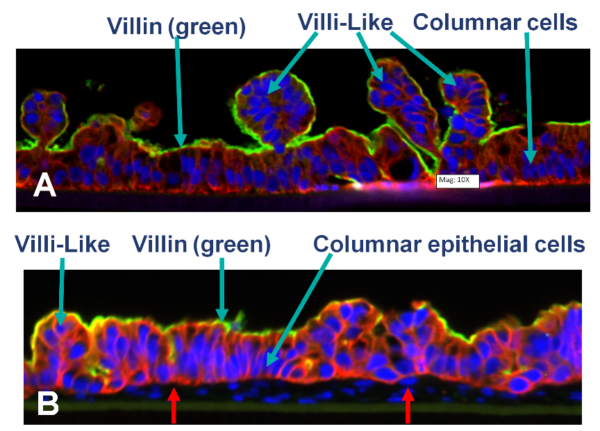
Confocal microscopic images showing: A) partial thickness (PT; epithelial cells only) and B) full-thickness (FT, containing fibroblasts and endothelial cells red arrow) small intestine tissue models cultured in microporous membrane, inserts under ALI conditions for 14 days.
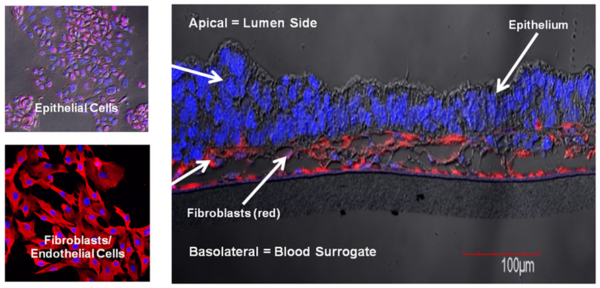
Normal human primary small intestine (SMI) epithelial cells, fibroblasts, and endothelial cells were expanded in monolayer culture and seeded onto transparent microporous membrane inserts to reconstruct the 3D SMI tissues. Injury was induced on the 3D tissues using 1 or 2 mm biopsy punches. The injured tissues were analyzed daily for epithelial restitution using phase contrast microscopy, confocal imaging of migrating epithelial cells (immuno-stained for cytokeratin 19) and fibroblasts (immuno-stained for vimentin), transepithelial electrical resistance (TEER) measurements to monitor recovery of epithelial barrier integrity, and H&E staining to examine the level of wound closure and re-epithelialization.
Following injury, TEER of the SMI tissues dropped from 160 Ω*cm2 (baseline) to 55 Ω*cm2. On day 1 after the injury, fibroblasts became more visible in the wound area and epithelial cells shouldering the wound began to migrate into the wounded area. Confocal and H&E imaging of injured tissues showed cooperation of fibroblasts and epithelial cells in the wound healing process. Wounded areas not resealed by epithelial cells were initially covered by fibroblasts. Overall, completion of wound healing was achieved in 4-6 days post-injury. On days 4-6: 1) the migrating epithelial cells resealed the wound and migrating epithelial cells re-polarized (confirmed by confocal imaging and H&E staining) and 2) tissue barrier returned to pre-injury, baseline levels (TEER).
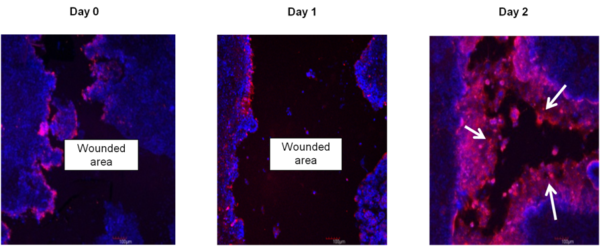
Immunohistochemistry showing restitution of organotypic 3D EpiIntestinal (SMI-100-FT) tissue after wounding with a needle tip (0.5 mm in diameter). Epithelial cells shouldering the wound migrate to reseal the injured tissue. Migrating epithelial cells express cytokeratin-19 (Red, see arrow); nuclei are stained with DAPI (Blue). Note: No treatment was applied to enhance the restitution process.
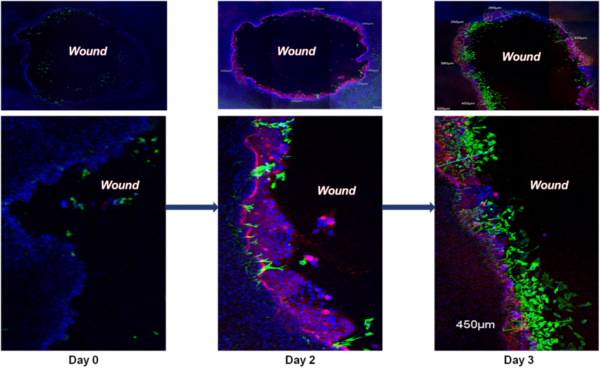
Wound Healing Progression: Immunohistochemistry showing restitution of organotypic 3D EpiIntestinal (SMI-100-FT) tissue after wounding with a needle tip (0.5 mm in diameter). Epithelial cells shouldering the wound migrate to reseal the injured tissue. Migrating epithelial cells express cytokeratin-19 (Red, see arrow); nuclei are stained with DAPI (Blue). Note: No treatment was applied to enhance the restitution process.
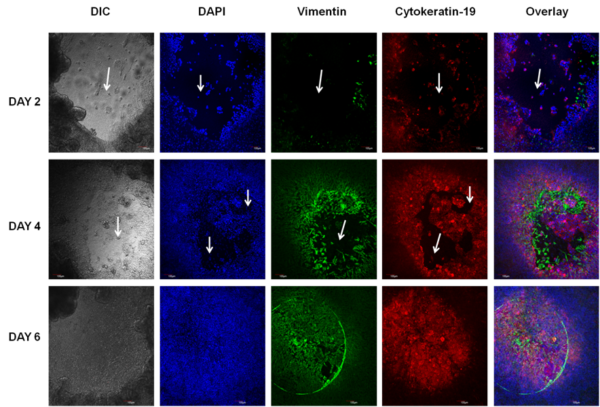
Wound Healing: Intestinal restitution of SMI-100-FT tissue model 3 days after wounding with a 2 mm biopsy punch. Migrating epithelial cells are stained for cytokeratin-19 (red), fibroblasts for vimentin (green), and nuclei are stained with DAPI (blue). On day 3, the fibroblasts are at the leading edge of resealing the wound.
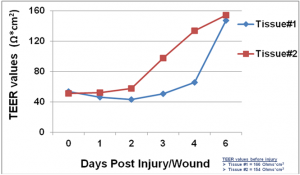
Barrier Recovery: TEER measurement showing recovery of epithelial barrier integrity following injury.
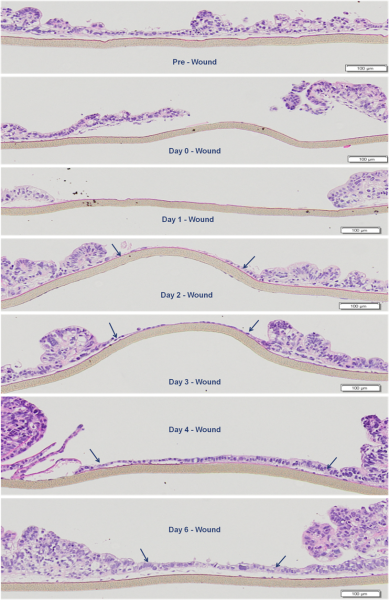
Wound Healing Progression: H&E staining showing epithelial restitution by the EpiIntestinal tissue model. Arrows indicate migrating epithelial cells shouldering the wound.
The newly developed SMI tissue model is useful for testing candidate drugs to treat diseases that are characterized by injuries of small intestine epithelial barrier.
For more information, view EpiIntestinal and References.
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**
Get MatTek offers and updates delivered to your inbox.