Get the latest
Get MatTek offers and updates delivered to your inbox.
The EpiOcular Eye Irritation Test predicts the acute eye irritation potential of a topically applied chemical or formulation by measurement of its cytotoxic effect of the EpiOcular cornea epithelial model. This in vitro hazard assessment allows for the identification of chemicals (substances and mixtures) not requiring classification and labeling for eye irritation or serious eye damage in accordance with UN GHS.
MatTek offers the EpiOcular Eye Irritation Test as a GLP or non-GLP service.
Protocol
| Test Model | EpiOcular |
| Replicates | N=2 tissues per test condition |
| Exposure Time | Liquids – 30 minute topical exposure to 50µl of test material per tissue Solids – 6 hour topical exposure to 50mg of test material per tissue |
| Assay Controls | Negative Control – Sterile deionized H2O Positive Control – Methyl Acetate |
| Endpoints | MTT Tissue Viability Assay |
| Data Delivery | % relative viability ± SD In vitro classification |
Download the EpiOcular Eye Irritation Test Protocol
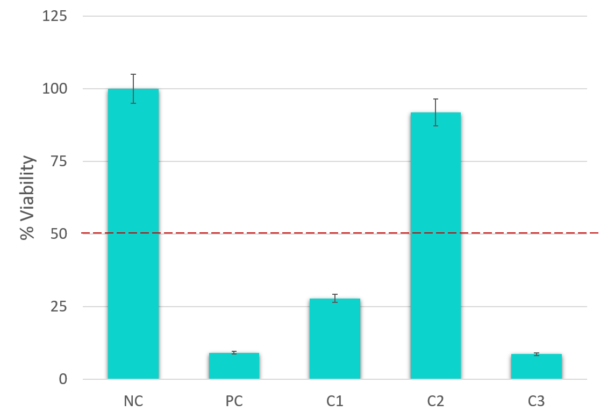
Data

References
Kaluzhny, Y., et al, (2015). EpiOcularTM Eye Irritation Test (EIT) for Hazard Identification and Labeling of Eye Irritating Chemicals: Protocol Optimization for Solid Materials and Extended Shipment Times. Altern Lab Anim. 2015 May;43(2):101-27.
OECD (2015), Test No. 492: Reconstructed human Cornea-like Epithelium (RhCE) test method for identifying chemicals not requiring classification and labeling for eye irritation or serious eye damage, OECD Publishing, Paris. DOI: http://dx.doi.org/10.1787/9789264242548-en
Request a Quote
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**
Get MatTek offers and updates delivered to your inbox.