Metabolic Stability
The HUREL micro liver coculture system is a robust and reliable method for human in vivo clearance prediction and metabolite detection of slowly metabolized drugs.
Background
- The hepatocyte stability assay is an in vitro method used to evaluate drug metabolism and predict hepatic clearance.
- While conventional models such as hepatocyte suspensions and liver microsomes provide only short-lived metabolic activity, they are limited for assessing low-turnover compounds.
- Primary hepatocytes in mono-culture can extend functional activity up to 24 hours, but with reduced enzymatic capacity.
- To overcome these challenges, Visikol employs the HUREL® micro liver models, which maintains stable phase I and II metabolic functions for over four weeks.
- This physiologically relevant system enables accurate measurement of parent drug depletion, half-life, and intrinsic clearance across multiple species, supporting reliable predictions of in vivo clearance, metabolite profiling, and interspecies comparisons in drug discovery.
General Procedure
General Procedure
- HUREL® micro liver models are prepared by co-culturing primary hepatocytes and stromal cells and cultured for one week prior to dosing compounds.
- Treatment with test compounds
- At each time point, samples are collected for LC-MS/MS analysis.
- Intrinsic clearance is calculated using the depletion of the parent compounds.
Protocol
| Cell model | HUREL® Primary Hepatocyte Micro Liver Models (Species: Human, Primate, Dog, Rat, Mouse, Minipig, Rabbit, etc) |
| Time points | t = 0, 4, 24, 48, 72, (168) hours after dosing (custom time points available) |
| Test Article Concentration | Single point assay (1 µM) (custom concentrations available) |
| Positive Controls | Diazepam (CYP3A, CYP2C19), Verapamil (CYP2D6, CYP3A, UGT) |
| Number of Replicates | 3 replicates per time condition |
| Method | Depletion of parent compounds |
| Analysis Method | LC-MS/MS |
| Test Article Requirements | 50 uL of 10 mM solution or equivalent amount of solid |
| Data Delivery | % remaining of parent compounds at each time point Half-life (t½) Intrinsic clearance (CLint) |
Data
Control Compounds
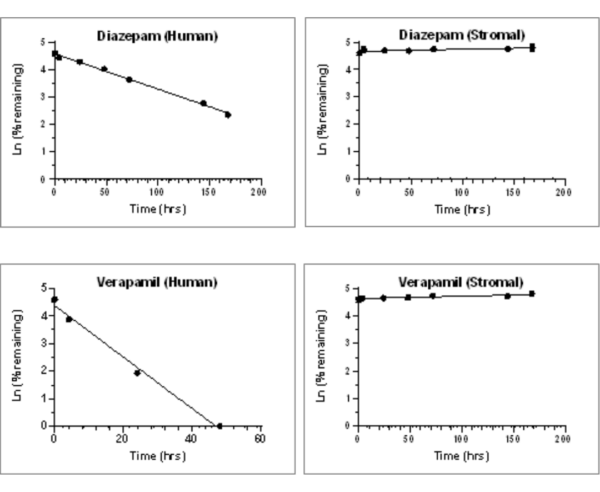
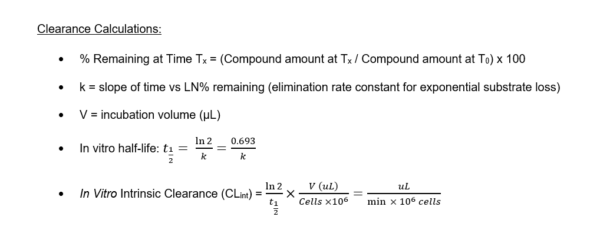
Figure 1. Clearance plots for control substrates in HURELhumanPool™ Micro Liver Model (Co-culture of primary hepatocytes/stromal cells) and stromal cells only.
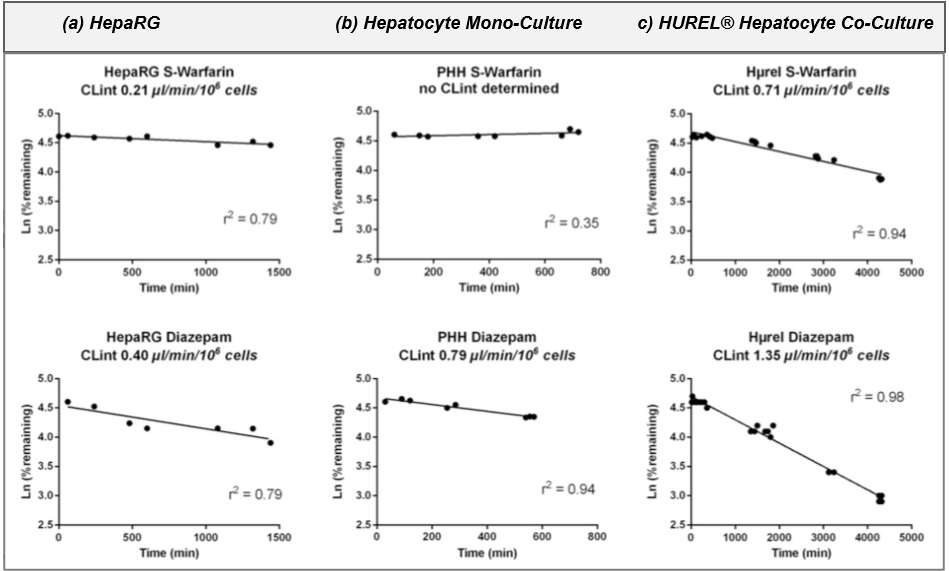
Figure 2. Clearance plots for reference controls (warfarin and diazepam) in (a) HepaRG, (b) Human hepatocyte mono-culture, and (c) HUREL® human hepatocyte co-culture (HURELhumanPool™ Micro Liver Model). Data from Bonn et al (2016), Drug Metab Dispos.
References
- Britta Bonn, Petter Svanberg, Annika Janefeldt, Ia Hultman and Ken Grime, Determination of Human Hepatocyte Intrinsic Clearance for Slowly Metabolized Compounds: Comparison of a Primary Hepatocyte/Stromal Cell Co-culture with Plated Primary Hepatocytes and HepaRG, Drug Metabolism and Disposition, April 2016, 44 (4) 527-533.
- Hultman, Charlotta Vedin, A. Abrahamsson, S. Winiwarter, M. Darnell, Use of HμREL Human Coculture System for Prediction of Intrinsic Clearance and Metabolite Formation for Slowly Metabolized Compounds, Molecular pharmaceutics, 14 July 2016

