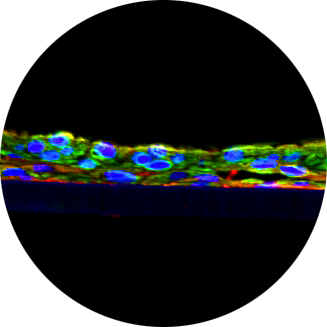
Oral Irritation
During product development of new oral care formulations, the oral irritation potential is evaluated to identify substances which may result in adverse reactions in the oral cavity. Understanding the irritation potential of oral care products and excipients is an important consideration in reducing potential risks.
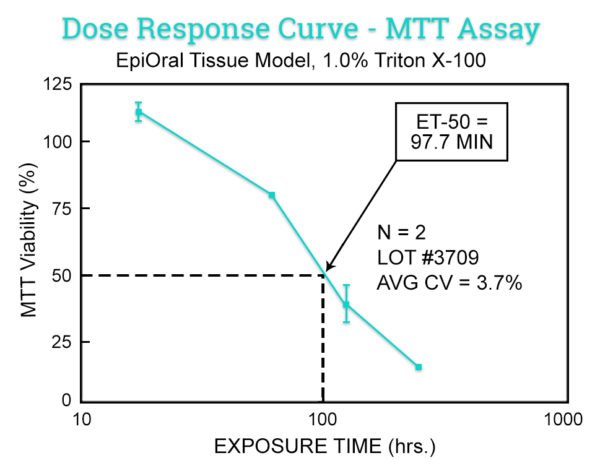
The in vitro EpiOral (buccal) and EpiGingival (gingival) MTT ET-50 assay is used as a screen to assign expected irritancy responses compared to benchmark controls based on the time to toxicity (ET-50) results obtained with EpiOral and EpiGingival. This assay is also used to rank order oral formulations according to their irritation potential.
Mattek offers the EpiOral and EpiGingival buccal irritation tests as non-GLP services.
Protocol
| Test Model | EpiOral or EpiGingival |
| Replicates | N=3 tissues per test condition |
| Exposure Time | 20, 60 and 120 minute topical exposure to 100µl of 1:1 diluted material per tissue |
| Assay Controls | Negative Control – Untreated |
| Endpoints | MTT Tissue Viability Assay |
| Data Delivery | ET-50 Values |
Download the EpiOral & EpiGingival Irritation Protocol
Data
References
Klausner, et al., (2007). Organotypic Human Oral Tissue Models for Toxicological Studies. Toxicology in Vitro. 2007 August;21(5):938-949.
Request a Quote
Thank you for requesting information about Mattek products! A representative will contact you shortly.
**If you would like to place an order for Mattek products, please contact Customer Service**