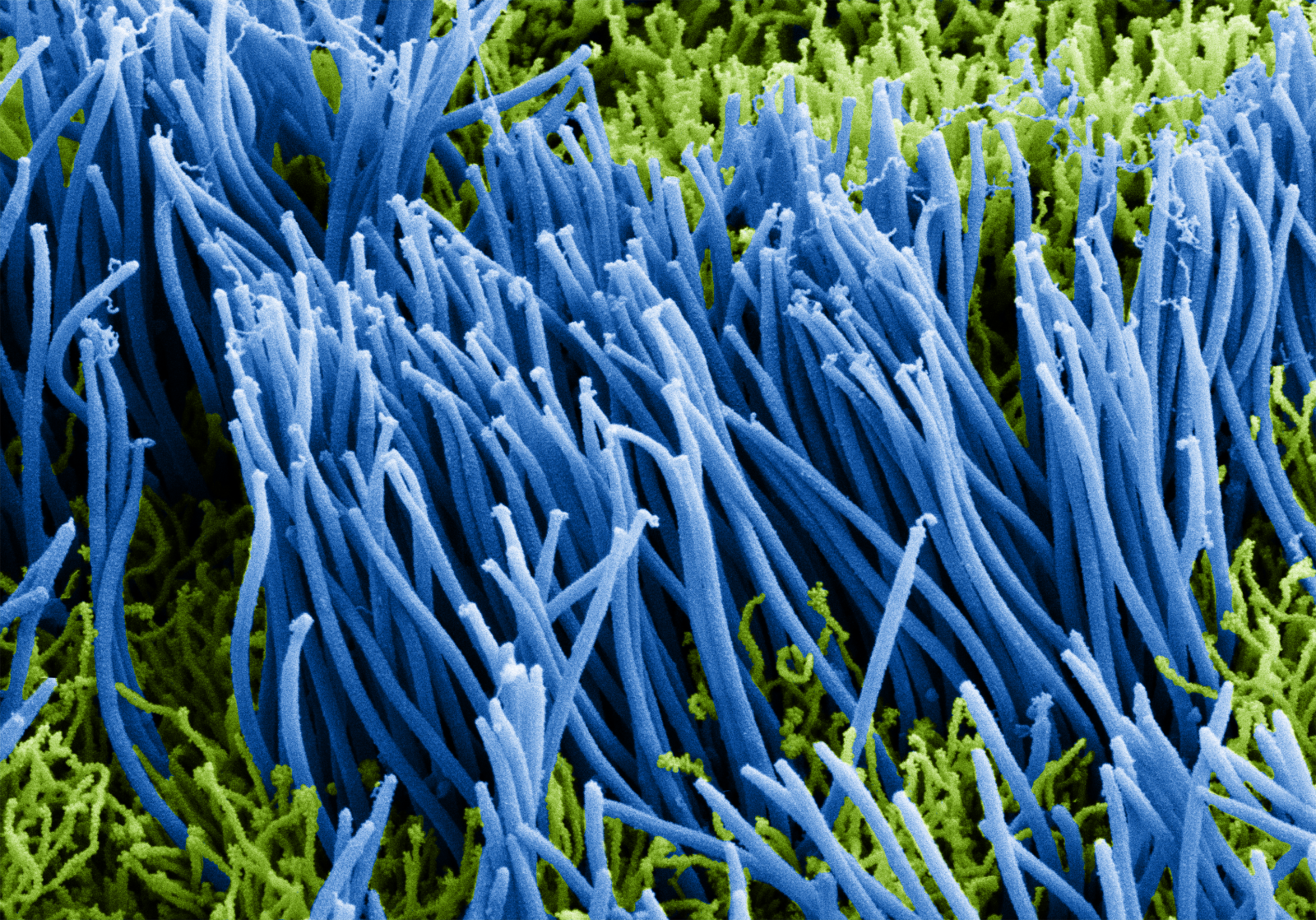
EpiAirway is a ready-to-use, 3D mucociliary tissue model consisting of normal, human-derived tracheal/bronchial epithelial cells also available as a co-culture system with normal human stromal fibroblasts (EpiAirwayFT). Cultured at the air-liquid interface (ALI), EpiAirway recapitulates the in vivo phenotypes of barrier, mucociliary responses, infection, toxicity responses and disease. With over 100 technical references available, EpiAirway is amenable to acute or long-term chronic studies across a wide range of highly predictive in vitro applications.
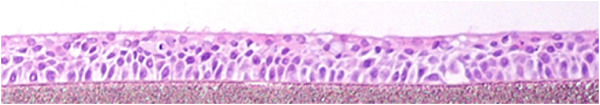
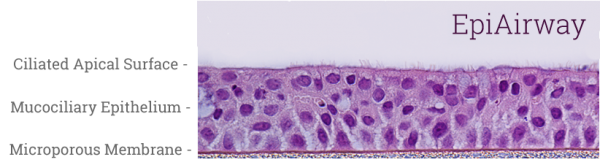
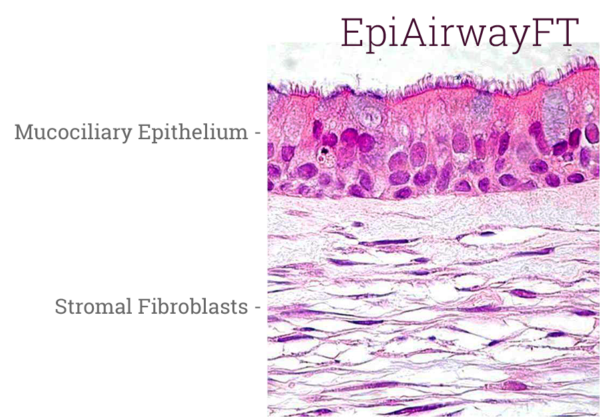
EpiAirway exhibits human relevant tissue structure and cellular morphology with high uniformity and reproducibility. Its 3D structure consists of organized Keratin 5+ basal cells, mucus producing goblet cells, functional tight junctions and beating cilia. EpiAirwayFT incorporates human fibroblasts in an extracellular stromal matrix ideal for inflammation and fibrosis research.
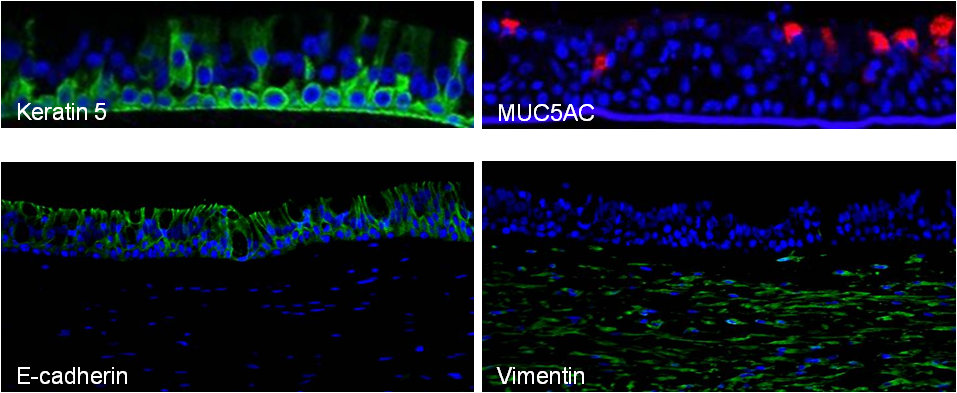
The EpiAirway 3D human tissue model is routinely utilized for a range of applications including safety & risk assessment, anti-viral research, inflammation & fibrosis and drug delivery. Simple protocols and the evaluation of early cellular endpoints allow scientists to acquire data in days, not weeks or months.
Virus Infection
Use EpiAirway to study respiratory virus attachment, replication, innate immune responses and anti-viral drug development.
Drug Delivery
Utilize existing transdermal permeation equipment or MatTek’s simple single insert permeation devices to assess API permeation and flux.
Inhalation Toxicology
Use EpiAirway (available in several formats for physiological exposure systems) to determine relative safety with the EpiAirway IC-75 assay.
Inhalation Toxicology Reference
Inflammation and Fibrosis
Employ EpiAirway’s diverse donor inventory of healthy and diseased donors (Asthma, COPD, Smoker, Goblet Cell Hyperplasia) to elucidate molecular pathways and assess therapeutic efficacy.
Tissue
Kit: A standard EpiAirway kit (AIR-100) consists of 24 tissues. (Tissue “kits” contain tissues, a small amount of culture medium, and plasticware; contact MatTek for specific kit contents)
Formats:
- 9mm & 22mm individual inserts – tissue culture substrate is chemically modified with a pore size of 0.4 μm
- 24mm, 12mm & 6.5mm Transwell inserts with a pore size of 0.4 μm
- 96-well HTS plates – tissue culture substrate is chemically modified with a pore size of 0.4 μm
Culture: Air-liquid interface
Histology: 3-4 cell layers – pseudostratified, mucociliary morphology and tissue structure
Lot numbers: Tissue lots produced each week are assigned a specific lot number. A letter of the alphabet is appended to the end of the lot number to differentiate between individual kits within a given lot of tissues. All tissue kits within a lot are identical in regards to cells, medium, handling, culture conditions, etc.
Shipment: At Room Temperature on medium-supplemented, agarose gels
Shipment day: Every Monday
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Thursday depending on location
Shelf life: Including time in transit, tissues may be stored at 4°C for up to 3 days prior to use. However, extended storage periods are not recommended unless necessary. In addition, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning)
Length of experiments: Tissue cultures can be continued for THREE (3) MONTHS or more with good retention of normal morphology. Tissues must be fed every other day with 5.0 ml of maintenance medium (AIR-100-MM). Cell culture inserts are placed atop washers (EPI-WSHR) or culture stands (MEL-STND) in 6-well plates to allow us of 5.0 ml. See technical reference #631.
Cells
Type: Normal human tracheal/bronchial epithelial cells (NHBE);
Genetic make-up: Single donor
Derived from: Healthy, non-smoker (alternate donors available upon request)
Alternatives: NHBE from Asthmatic, COPD and Smoker donors
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma
Medium
Base medium: Dulbecco’s Modified Eagle’s Medium (DMEM)
Growth factors/hormones: Epidermal growth factors and other proprietary factors
Serum: None
Antibiotics: Gentamicin 5 µg/ml (10% of normal gentamicin level)
Anti-fungal agent: Amphotericin B 0.25 µg/ml
pH Indicator: Phenol red
Other additives: Proprietary
Alternatives: Phenol red-free, antibiotic-free, anti-fungal-free medium and tissues are available. Agents are removed at least 3 days prior to shipment.
Assay medium: AIR-100-ASY
Maintenance medium: AIR-100-MM
Quality Control and Sterility
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment
End-use testing: Transepithelial electrical resistance (TEER) of each EpiAirway lot is measured using an EVOM2 epithelial Volthommeter and Endohm electrode chamber (World Precision Instruments). A minimum TEER of 300 Ohm • cm2 is required for QC release.
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service
Notification of lot failure: If a tissue lot fails our QC or sterility testing, the customer will be notified and the tissues will be replaced without charge when appropriate. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances, TEER failures will be notified by Wednesday 5 p.m.; sterility failures will be notified within 8 days of shipment)
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**


