Get the latest
Get MatTek offers and updates delivered to your inbox.
In bringing a therapuetic through clinical trials, one of the leading contributors to clinical failure is liver toxicity causing 15% of failures. It is for this reason that there has always been significant interest in developing inexpensive and moderate throughput in vitro models which are capable of predicting clinical drug-induced liver injury (DILI). MatTek has leveraged its decades of experience in developing highly reproducible advanced cell culture models to launch EpiLiver.
Tissue morphology has been characterized by histology and albumin expression has been evaluated by immunohistochemistry and ELISA. The model shows recapitulation of the liver’s structure with 3D columnar hepatocyte tissue formation and hexagonal cellular structure (topical view imaging). Albumin production (immunohistochemistry), and albumin release to the basolateral and apical sides (ELISA) has also been demonstrated.
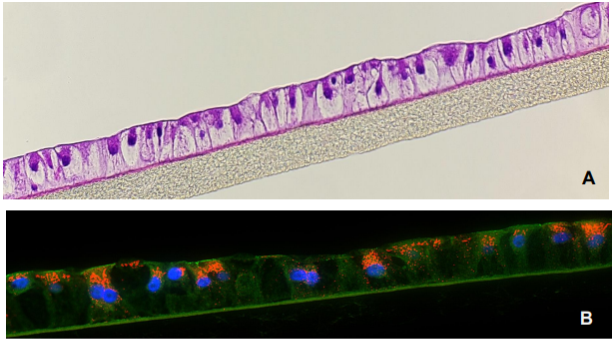
Figure 1. H&E-stained histological cross-section of the 3D human liver tissue model showing the hepatocyte polarization and stratification (A). Immunohistochemistry of albumin (orange) production is shown in (B). Tissues were grown on collagen coated underlying microporous membrane support (pore diameter= 0.4 um).
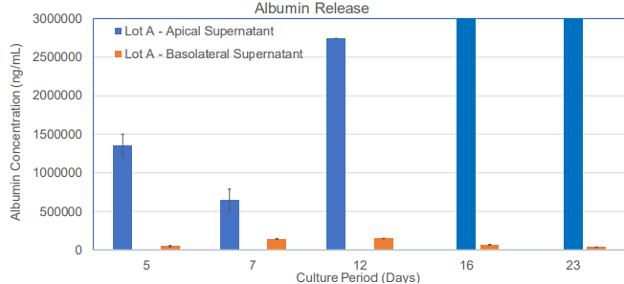
Figure 2. Albumin release by the 3D human liver tissue model at different time points of the culture period.
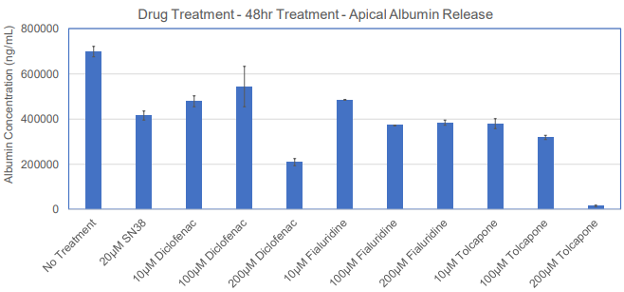
Figure 3. Reduction of albumin release following treatment of the 3D human liver tissue model with different drugs dosed at different concentrations.
Changes in gene expression levels for drug metabolizing enzymes associated with first pass metabolism have also been assessed via qPCR over a period of 23 days. qPCR results demonstrate high level expression of enzymes involved in drug metabolism such as CYP2C8, CYP2C9, CYP2C18, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A5, CYP3A7, and CYP4A11. Both Phase I and Phase II enzymes were expressed by the differentiated liver tissue model.
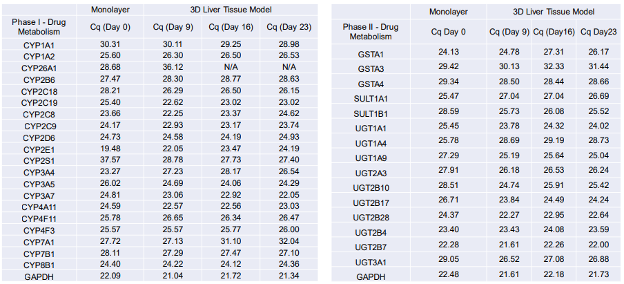
Table 1. qPCR Results (RT2 Profiler PCR Array Kit) showing Phase I and II drug metabolizing enzyme gene expression levels by the 3D human liver tissue model.
The utility of the tissue model for liver toxicity studies has been demonstrated by dosing the reconstructed liver tissue with 100 µM of 5 model drugs (SN38, Bosentan, Diclofenac, Fialuridine, and Tolcapone) that are known to have adverse effects on liver in humans. Outcome measurements for liver toxicity include increased levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) release, two biomarkers with clinical relevance in liver functionality tests. Repeated application of Fialuridine, a drug intended for hepatitis B treatment that was abruptly terminated due to induction of liver failure or causing of severe liver toxicity during clinical trials, showed an increase in ALT and AST levels in a time-dependent manner at days 5 and 7, which is indicative of drug induced liver injury (DILI). The positive control, SN38, a metabolite of the cancer drug Irinotecan, also shows an increase in ALT and AST levels.
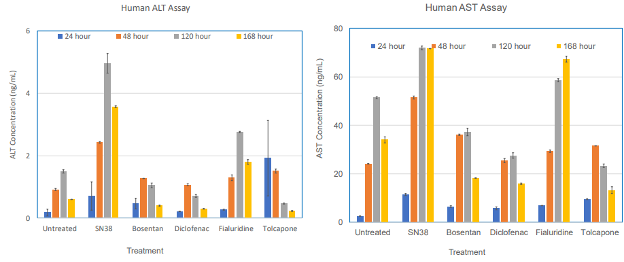
Figure 4. Acute exposure of the 3D human liver tissue to drugs known to induce liver toxicity showed increases in ALT and AST release, two biomarkers clinically used for liver function test.
Development of this novel 3D human liver tissue model using primary adult hepatocytes provides researchers the opportunity to screen the potential liver toxicity of candidates with a highly relevant model that are in the drug development pipeline. Such a model will allow formulation scientists to identify adverse effects of therapeutic candidates early in the drug development process. This model will also reduce animal use for experimentation.
We have designed the EpiLiver model to be applicable to a broad range of applications from evaluating drug metabolism (DMPK) to assessing drug induced liver injury which accounts for 15% of preclinical and clinical stage failures.
Drug-Induced Liver Injury (DILI)
The highly relevant EpiLiver model is capable of assessing and predicting in vivo liver toxicity.
Drug Metabolism
Understanding how drugs are metabolized is crucial to understanding the route of administration and any toxicity liabilities associated with metabolism.
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**
Get MatTek offers and updates delivered to your inbox.