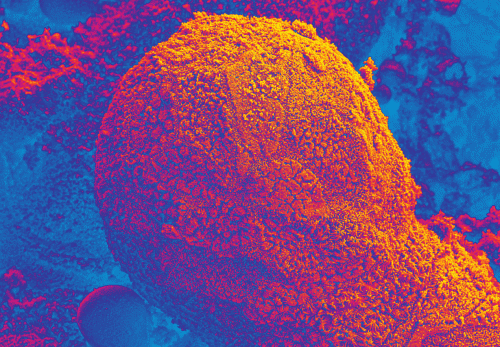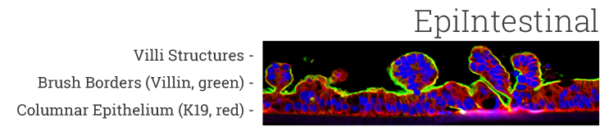
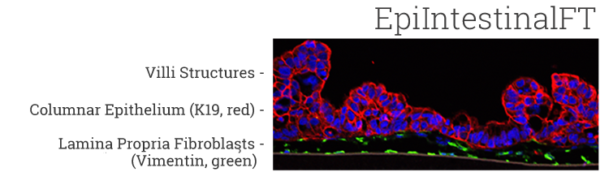

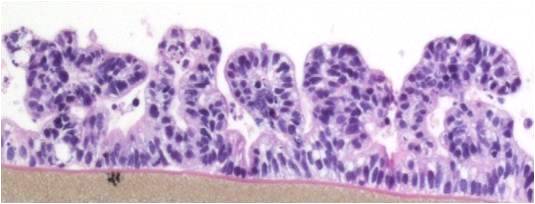
The EpiIntestinal tissue model (SMI-100) offers numerous advantages versus currently available preclinical testing models such as Caco-2. The human cell-based 3D model incorporates enterocytes, paneth cell, M cells, tuft cells and intestinal stem cells into a highly differentiated, polarized epithelium. The EpiIntestinalFT tissue (SMI-100-FT) includes an underlying lamina propria containing normal human intestinal fibroblasts and is useful to study inflammation, wound repair, innate immune responses, and fibrosis. Both tissues are cultured at the air-liquid interface (ALI) to allow physiological (luminal) exposure conditions. EpiIntestinal recapitulates many aspects of normal intestinal function including barrier, metabolism, inflammatory and toxicity responses, similar to native human intestinal tissue.
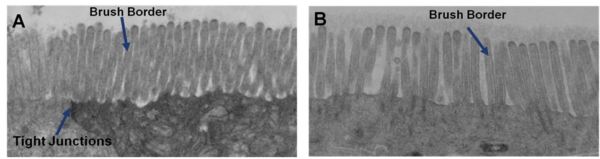
Important features of EpiIntestinal include the presence of functional tight junctions and brush borders at the apical tissue surface. In vivo, brush borders are in direct contact with luminal contents and include at least 22 metabolic enzymes and 19 drug transporters. EpiIntestinal tight junctions and brush borders closely mimic human structure and physiology.
The EpiIntestinal 3D human tissue model is routinely utilized for screening pharmaceuticals for a range of endpoints including GI toxicity and inflammation, drug metabolism, drug permeation, fibrosis, and intestinal infection. Straightforward protocols and the evaluation of early molecular and cellular endpoints often allow scientists to acquire data in days, not weeks or months.
GI Toxicity
Use EpiIntestinal for a highly predictive assay to evaluate GI toxicity of pharmaceuticals.
Inflammation and Fibrosis
Utilize EpiIntestinal’s highly controlled in vitro culture conditions to study intestinal inflammation, wound repair, and fibrosis and assess therapeutic effects.
Intestinal Inflammation Reference
Drug Delivery & Metabolism
Utilize EpiIntestinal for highly predictive and human-relevant drug delivery. Assess API bioavailability (influx) and efflux transport, drug metabolism, and drug-drug interactions. EpiIntestinal can be co-cultured with other physiological systems (e.g. liver cells) in microfluidic systems to examine the effect of drug metabolites on GI toxicity or to predict drug permeation/absorption via the “luminal” side of the tissue.
Intestinal Drug Delivery Reference
Wound Repair/Epithelial Restitution
Use EpiIntestinalFT to better understand mechanisms of wound healing in the gut and to evaluate therapeutic effects.
Intestinal Restitution Reference
Intestinal Infection
Utilize EpiIntestinal to study GI tract infection, host-pathogen interactions, and innate immune responses.
Browse our reference library to learn more about how researchers have used our EpiIntestinal tissue in these areas of study.
Tissue:
Kit: SMI-100 and SMI-100-FT EpiIntestinal kits consist of 24 individual tissues. SMI-196 or SMI-196-FT EpiIntestinal kits consist of 96 tissues in a high throughput screening plate. Each kit contains tissues, culture medium to support experiments for 24 hours, and plasticware. Please contact MatTek for specific kit contents.
Formats:
- SMI-100 and SMI-100-FT: Individual tissues cultured in cell culture inserts.Dimensions: 9 mm tissue diameter (surface area= 0.6 cm2 ), underlying microporous membrane pore size = 0.4 μm
- SMI-196 and SMI-196-FT: Tissues grown in 96-well high throughput screening plate. Dimensions: 0.19 cm tissue diameter (surface area= 0.11 cm2). Underlying microporous membrane pore size =0.4 μm
Culture: Air-liquid interface
Lot numbers: Tissue lots produced each week are assigned a specific lot number. A letter of the alphabet is appended to the end of the lot number to differentiate between individual sets of 24 tissues within a given production lot of tissues. All tissue kits within a production lot are identical in regards to cells, medium, handling, culture conditions, etc.
Shipment: At 4°C on medium-supplemented, agarose gels
Shipment day: Every Monday
Delivery: Tuesday morning via FedEx priority service (US). Outside US: Tuesday-Wednesday depending on location.
Shelf life: Including time in transit, tissues may be stored at 4°C for up to 2 days prior to use. However, the best reproducibility will be obtained if tissues are used consistently on the same day, e.g. Tuesday afternoon or following overnight storage at 4°C (Wednesday morning).
Length of experiments: Tissue cultures can be continued for ONE (1) MONTH or more with good retention of normal morphology. Tissues must be fed every other day with 5.0 ml of maintenance medium (SMI-100-MM). Cell culture inserts are placed atop washers (EPI-WSHR) or culture stands (MEL-STND) in 6-well plates to allow use of 5.0 ml.
Cells:
Type: Primary human small intestinal epithelial cells (HSIE)
Genetic make-up: Single donor
Derived from: Healthy adult donor
Alternatives: Alternate donors available upon request
Screened for: HIV, Hepatitis-B, Hepatitis-C, mycoplasma
Medium:
Base medium: Dulbecco’s Modified Eagle’s Medium (DMEM)
Growth factors/hormones: Epidermal growth factor and other proprietary factors
Antibiotics: Gentamicin 5 µg/ml
Anti-fungal agent: Amphotericin B 0.25 µg/ml
pH Indicator: Phenol red
Other additives: Proprietary
Alternatives: Phenol red-free, antibiotic-free, anti-fungal-free medium and tissues are available. Agents are removed at least 3 days prior to shipment.
Quality Control and Sterility:
Visual inspection: All tissues are visually inspected and if physical imperfections are noted, tissues are rejected for shipment
Sterility: All media used throughout the production process is checked for sterility. Maintenance medium is incubated with and without antibiotics for 1 week and checked for sterility. The agarose gel from the 24-well plate used for shipping is also incubated for 1 week and checked for any sign of contamination
Screening for pathogens: All cells are screened and are negative for HIV, hepatitis B and hepatitis C using PCR. However, no known test method can offer complete assurance that the cells are pathogen free. Thus, these products and all human derived products should be handled at BSL-2 levels (biosafety level 2) or higher as recommended in the CDC-NIH manual, “Biosafety in microbiological and biomedical laboratories,” 1998. For further assistance, please contact your site Safety Officer or MatTek technical service
Notification of lot failure: If a tissue lot fails QC or sterility testing, the customer will be notified and the tissues will be replaced without charge. Because our QC and sterility testing is done post-shipment, notification will be made as soon as possible (Under normal circumstances, ET-50 failures will be notified by Thursday 5 p.m.; sterility failures will be notified within 8 days of shipment)
New Orders
To ensure that the most appropriate products and accessories for your application are included with your initial order, MatTek strongly recommends a complimentary consultation with one of our highly trained Technical Specialists. In the US, please contact Alex Armento to order EpiIntestinal products. In the EU, please contact Dr. Jan Markus.
Alex Armento
MatTek Corporation
aarmento@mattek.com
T: (508) 881-6771 Ext. 242
F: (508) 879-1532
Dr. Jan Markus
MatTek In Vitro Life Science Laboratories
Bratislava, Slovak Republic
jmarkus@mattek.com
T: +421-2-3260-7403
F: +421-2-3260-7404
All Other Orders
Please contact Customer Service
Customer Service
MatTek Corporation
T: +1 (508) 881-6771
F: +1 (508)-879-1532
information@mattek.com