Get the latest
Get MatTek offers and updates delivered to your inbox.
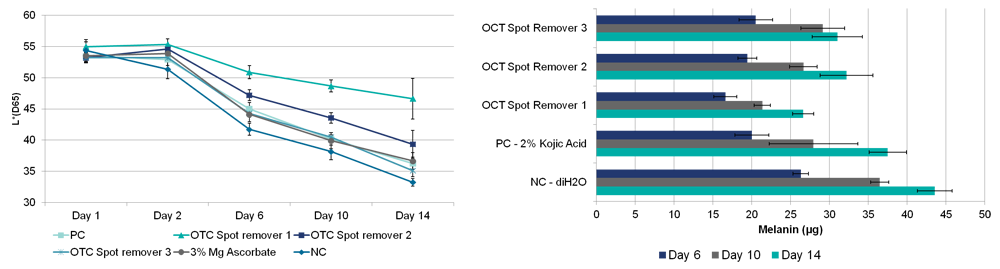
The MelanoDerm Skin Brightening Assay provides invaluable in vitro data as an early screening tool for raw materials to reduce costs and increase the chances of a formulation’s success prior to clinical trials. Cosmetics manufacturers continually develop safer and more effective skin brightening products for their customers struggling with melasma and post-inflammatory hyperpigmentation. Evaluate the efficacy of cosmetic and/or pharmaceutical skin care formulations that are used to combat skin pigmentation disorders with MelanoDerm.
MatTek offers MelanoDerm Skin Brightening/melanogenesis assay as a non-GLP service.
Protocol
| Test Model | MelanoDerm |
| Replicates | N=8 tissues per test condition |
| Exposure Conditions | 3 treatments/week for 2 weeks with 25µl of test material and controls |
| Assay Controls | 2% Kojic Acid (positive control), DI H2O (negative control) |
| Endpoints | Macroscopic analysis, Histological analysis, Quantitative Melanin Assay |
| Data Delivery | Histological images and analysis, Melanin Assay Analysis, Macroscopic photos skin brightening potential |
Download the MelanoDerm Skin Brightening Assay Protocol
Data

References
Request a Quote
Get MatTek offers and updates delivered to your inbox.