Get the latest
Get MatTek offers and updates delivered to your inbox.
Skin irritation refers to the production of reversible damage to the skin following the application of a test substance. Understanding the potential of topically-applied chemicals or formulations to induce skin irritation (hazard) is an important consideration in their safety evaluation.
The EpiDerm Skin Irritation Test was developed and designed to predict skin irritation potential of neat test substances in the context of identification and classification of skin irritation hazard according to the EU classification system (R 38 or no label) and the UN GHS system. The EpiDerm Skin Irritation Test allows for the discrimination between irritants of category 2 and non-irritants.
MatTek offers the EpiDerm Skin Irritation Test as a GLP or non-GLP service.
Protocol
| Test Model | EpiDerm |
| Replicates | N=3 tissues per test condition |
| Exposure Time | 1 hour topical exposure to 30µl or 25mg of test material per tissue |
| Test Article Quantity | 1ml or 1g |
| Assay Controls | Negative Control – Sterile DPBS Positive Control – 5% SDS |
| Endpoints | MTT Tissue Viability Assay |
| Data Delivery | % relative viability ± SD In vitro classification |
Download the EpiDerm Skin Irritation Test Protocol
Data
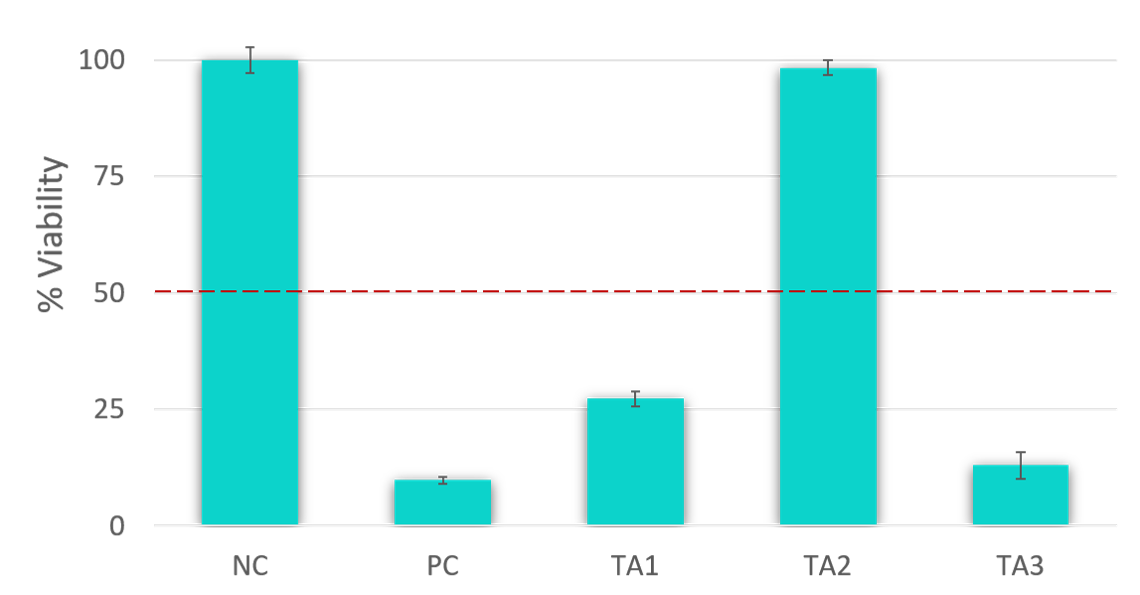
The determination of skin irritation potential of test materials is based on tissue viability. According to the EU and GHS classification (R38 / Category 2 or no label), an irritant is predicted if the mean relative tissue viability of triplicate tissues exposed to a test material is reduced below 50% of the mean viability of the negative controls.
| in Vitro Result | in Vivo Prediction |
|---|---|
| Mean tissue viability < 50% | Irritant (I), (R38 or GHS Category 2) |
| Mean tissue viability > 50% | Non-irritant (NI) |
References
Download the OECD Test Guideline 439
Reference 809 – Application of MatTek In Vitro Reconstructed Human Skin Models for Safety, Efficacy Screening, and Basic Preclinical Research
Request a Quote
Thank you for requesting information about MatTek products! A representative will contact you shortly.
**If you would like to place an order for MatTek products, please contact Customer Service**
Get MatTek offers and updates delivered to your inbox.